Oxidation of Copper Nickel alloy
Experimental data and modelling of Oxidation of CuNi alloy
The air oxidation of a CuNi alloy wire has been shown to fit an Antione Vapor Pressure equation. The derived equation correctly predicts the lower limit for oxidation of Cu55Ni45 alloys is air (650 °C) and is an excellent predictor for the rate of oxidation of this alloy wire in air. It is also shown that the density of the oxide formed is 33.3% +- 3.16.
The facility of Cu55Ni45 alloys as a basis for the production of effective catalyst for the evolution of anomalous excess heat in a hydrogen atmosphere was demonstrated by Francesco Celani in 2012. The alloy, in a wire form, requires activation by a proprietary cyclic oxidation process followed by reduction in a hot hydrogen atmosphere.
This page documents experimental data and numeric modelling for the oxidation rate and porosity of oxide formed on a 0.721mm dia alloy, Cuprothal 49 (Cu 54.5, Ni 44.0, Mn 1.0, Fe 0.5 % composition).
Experimental procedure
Sets of 6cm lengths of the test wire were cut and bent into a shape continent for their rapid removal from a kiln. In sets of 12 these wires were placed on a refractory wool support and loaded into a kiln preheated to the desired temperature. Tests had confirmed that the kiln could be opened and wire sets inserted or individual wires removed with minimal effect on the kiln temperature -- upon removal of a sample wire the kiln temperature recovered within 30 seconds. In addition the remaining wires appeared to suffer little temperature loss in the brief time the kiln was open.
Individual wires in a set were removed from the kiln at regular intervals and rapidly cooled in moving air. The cooling time to ambient was under 30 seconds. Once removed the total wire diameter was measured with a digital micrometer. After ensuring a good sampling of the exterior diameter the oxide layer was easily removed by mechanical stressing to reveal the un-oxidized and undamaged core wire which was also measured.
Data and analysis provided by Dr Mark Snoswell, CTO Chava. All inquiries should be directed to
Copyright Chava 2012. No copying permitted – refer all links back to this web page.
Results - growth of oxide layer on 0.712mm dia wire
The accompanying graph shows the results for measurements on the accumulation of oxide layer on the wire at 4 different temperatures: 900,950,1000 and 1050 °C. The graph also shows fitted lines generated by a single equation as detailed below.
Test were done at up to 1100 °C. Data for temperature over 1050 °C is not shown here as above 1050 °C the oxide formed began to sinter resulting in rough surfaces and great difficulty in removal the sintered oxide from the wire core.
A number of tests were repeated over a significant time period (months) with no change in oxidation behavior observed. Tests were also done in a quartz tube furnace under conditions of flowing air with
continuous monitoring of the wire resistance as a further indicator of the progression of oxidation. Again the results indicate that the progression of oxidation of the wire is a very robust and highly repeatable process. 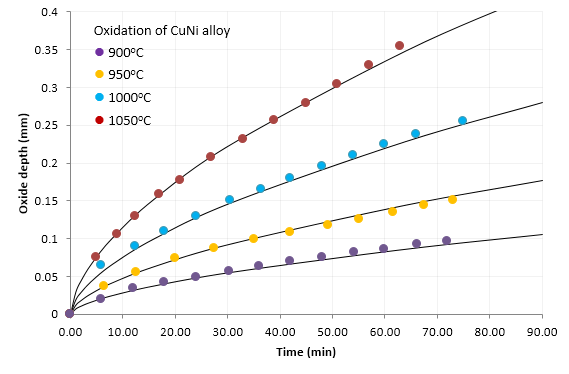
Results - porosity of oxide layer
The accompanying graph shows the results for measurements on the porosity of the oxide layer on the wire at 3 different temperatures: 900,950 and 1050 °C.
Measurement of the volume of alloy lost from the core permits a calculation of the expected volume o fa 100% dense oxide from published data on the density of Cu and Ni oxides. This anticipated volume is then compared with the actual volume of the oxide layer observed to arrive at a density of the oxide, The accompanying graph represents this as a porosity. It is difficult to reduce the noise of this derived measurement. However, considering all the experiments collectively there was no evident trend in oxide density.
Although not shown here, subsequent reduction of the oxide layer does not result in any physical shrinkage of the overall wire diameter. As a consequence the porosity of the subsequently reduced layer can be estimated to be 45.8%.
Results - numeric modeling
The accompanying graph shows the oxide layer formation predicted by the numeric model developed for temperatures between 650-1050 °C.
A 2 variable surface function was fitted using Lab Fit software. The first graph at the top of the page showed the fitted curves for the 4 data sets shown. The graph here shows the predicted data for a much wider range of temperatures than those sampled experimentally. It is known that CU55Ni45 alloys do not oxidize below 650 °C -- which is in good agreement with the predictions from the numerical modelling.
The equation derived is:
Oxd = exp(2.77612 - (4388.85 / tC) + (0.297535 * ln(tm))
where...
Oxd = oxide layer depth in mm
tC = temperature in °C
tm = time in min

Photos

For reliable measurements in the micron accuracy range the ambient temperature should be constant. Fortunately most of the critical analysis is done on the difference of two measurements taken at the same time. We also take a number of diametrical measurements ensuring that the same pressure is always applied across the micrometer jaws. With care, repeatable, micro meter measurement accuracy is routinely achievable.

Note the copper color of the core. During oxidation the copper is much more mobile than the Nickel and it migrates to the surface. This is also seen in the color of the wire after reduction (not shown here) -- the surface appears like pure copper. As CA0 Zhong-qiu et al. reported "Typical double-layered scales are produced, which consist of a CuO outer layer and an inner layer containing a mixture of Cu20 and NiO with many pores".

Here we can see the difference between the virgin Cuprothal 49 wire and the oxidized wire. The oxide layer is quite strong but can be fractured with moderate pressure to reveal the core. Below 1050 °C the oxide layer does not adhere to the core. Above 1050 °C the oxide begins to sinter resulting in it sticking to the core and to a rough outer surface. An excellent reference is: Air oxidation of Cu-50Ni and Cu-70Ni alloys at 800 °C.
Copyright © 2026. Chava Science.