Working at General Electric Company
Langmuir first entered the Research Laboratory of the General Electric Company in the summer of 1909, expecting that by fall he would return to Stevens Institute, where he had been teaching chemistry. Instead of assigning him to any definite work, Doctor Whitney who was heading the Lab, suggested that he spend several days in the various rooms of the laboratory, becoming familiar with the work that was carried out. Dr. Whitney also asked him to let him know what he found of most interest as a problem for the summer vacation. A large part of the laboratory staff was busily engaged in the development of drawn tungsten wire made by the then new Coolidge process. A serious difficulty was being experienced in overcoming the offsetting of the filaments. This was a kind of brittleness which appeared only when the lamps were run on alternating current. Out of a large number of samples of wire, three had accidentally been produced which gave lamps that ran equally well with alternating and with direct current, but there was no explanation as to what had made these wires so good. It seemed to Langmuir that there was one factor that had not been considered, which was, that the offsetting possibly was due to impurities in the wire in the form of gases. He therefore suggested to Doctor Whitney that he would like to heat various samples of wire in high vacuum and measure the quantities of gas obtained in each case. In looking through the laboratory he had been particularly impressed with the remarkably good methods that were used for exhausting lamps. These methods were, he thought, far better than those known to scientific research workers at the time. His desire to become more familiar with these methods was undoubtedly one of the factors that led him to select for his first research an investigation of the gas content of wires. After starting the measurements that he had planned, he found that the filaments gave off surprisingly large quantities of gas. Within a couple of weeks he realized that something was entirely wrong with his apparatus, because from a small filament in a couple of days he obtained a quantity of gas which had, at atmospheric pressure, a volume 7000 times that of the filament from which it appeared to have come; and even then there was no indication that this gas evolution was going to stop. At the time one could find in the literature - for example in J. J. Thomson's book on the "Conduction of Electricity through Gases" - many statements that metals in vacuum give off gases almost indefinitely, and that it is impossible to free metals from gas by heating. Still he thought that 7000 times its own volume of gas was an entirely unreasonable amount to obtain from a filament. He spent most of the summer trying to find where this gas came from, and did never investigate the different samples of wire to see how much gas they contained. To any other researcher, it would have been much more logical if he had dropped the work as soon as he found that he would not be able to get useful information on the off-setting problem by the method that he had employed. Instead Langmuir continued working. What he really learned during that summer was that glass surfaces which have not been heated a long time in vacuum slowly give off water vapor, and this reacts with a tungsten filament to produce hydrogen. At the same time, the vapors of Vaseline from a ground-glass joint in the vacuum system give off hydrocarbon vapors, which produce hydrogen and carbon monoxide. That summer's work was so interesting to him that he dreaded to return to the comparative monotony of teaching, and gladly accepted Doctor Whitney's offer to continue working at the laboratory. No definite program of work was laid down. He was given at first one assistant and then others to continue experiments on the sources of gas within vacuum apparatus, and a study of the effects produced by the introduction of various gases into tungsten filament lamps. The truth is that he was merely curious about the mysterious phenomena that occurred in these lamps. Doctor Whitney had previously found that gases had a habit of disappearing in lamps, and no one knew where they went to, so Langmuir wanted to introduce each different kind of gas that he could lay his hands on into a lamp with a tungsten filament and definitely find out what happened to that gas.
A better lamp
It was the universal opinion among the lamp engineers with whom he came in contact that if only a much better vacuum could be produced in a lamp a better lamp would result. Doctor Whitney, particularly, believed that every effort should be made to improve the vacuum, for all laboratory experience seemed to indicate that this was the hopeful line of attack on the problem of a better lamp. However, Langmuir really didn't know how to produce a better vacuum, and instead proposed to study the bad effects of gases by putting gases in the lamp. He hoped that in this way he would become so familiar with the effects of these gases that he could extrapolate to zero gas pressure, and thus predict, without really trying it, how good the lamp would be if they could produce a perfect vacuum. This principle of research he found extremely useful on many occasions. When it is suspected that some useful result is to be obtained by avoiding certain undesired factors, but it is found that these factors are very difficult to avoid, then it is a good idea to increase deliberately each one of these factors in turn so as to exaggerate their bad effects, and thus become so familiar with them that one can determine whether it is really worth while avoiding them. For example, if you have in lamps a vacuum as good as you know you can produce, but suspect that the lamps would be better if you had a vacuum, say, 100 times as good it may be the best policy, instead of attempting to devise methods of improving this vacuum, to spoil the vacuum deliberately in known ways, and you may then find that no improvement in vacuum is needed or just how much better the vacuum needs to be. During these first few years, while he was thus having a good time satisfying his curiosity and publishing scientific papers on chemical reactions at low pressures, he frequently wondered whether it was fair that he should spend his whole time in an industrial organization on such purely scientific work, for he didn't really see what applications could be made of it. Several times he talked the matter over with Doctor Whitney, saying that he could not tell where this work was going to lead them. Whitney replied that it was not necessary, as far as he was concerned, that it should lead anywhere. He would like to see him continue working along any fundamental lines that would give them more information in regard to the phenomena taking place in incandescent lamps, and that he should feel himself perfectly free to go ahead on any such lines that seemed of interest to him. For nearly three years he worked in this way with several assistants working for him before any real application was made of any of his work. In adopting this broad-minded attitude Doctor Whitney showed himself to be a real pioneer in the new type of modern industrial research known as Free Research, and thus made Langmuir the first scientist in modern history to be given the tools and resources for free research. For his study of the effect of gases, he had to devise new types of vacuum apparatus. He needed particularly to be able to analyze the small quantities of gas that existed in the tungsten lamp. With some of this special apparatus he was able to make a practically complete quantitative analysis of an amount of gas which would occupy about 1mm3 at atmospheric pressure. In this sample of gas they could determine the percentages of oxygen, hydrogen, nitrogen, carbon dioxide, carbon monoxide, and the inert gases. In regard to the behavior of the different gases which he introduced into the lamp bulb, he found that no two gases acted alike. For example, Oxygen attacked the filament and formed tungstic oxide, W03. That seemed simple enough, but the kinetics of the reaction presented many features of considerable scientific interest.
The effect of Hydrogen and problems with water
In studying the effect of hydrogen he observed very peculiar phenomena. A limited amount of hydrogen disappeared and became adsorbed on the bulb, where it remained in a chemically active form, which was capable of reacting with oxygen at room temperature even long after the tungsten filament had been allowed to cool. This suggested hydrogen atoms and seemed to confirm some conclusions that Langmuir had already drawn from observations on the heat losses from tungsten filaments in hydrogen at atmospheric pressure. In making squirted tungsten filaments, and sometimes in cleaning the drawn wire, filaments were heated in this manner in hydrogen. Because tungsten filaments melt at a temperature 1500o above the melting point of platinum, it seemed to him that tungsten furnished a tool of particular value for the scientific study of phenomena in gases at high temperatures. From his work on lamps he knew approximately the relation between the electrical resistance of tungsten wire and its temperature, and could thus use a tungsten wire as a kind of resistance thermometer. By connecting a voltmeter and an ammeter to the tungsten filament which was being heated in hydrogen, he could determine the temperature as well as find the heat loss from the filament in watts. He wanted to see if anything abnormal happened when the temperature was raised to the extremes which were only possible with tungsten. The results greatly interested him, for they showed that the energy loss through the gas, was proportional to the square of the temperature up to about 1800oK., and increased at a much higher rate above that, until at the highest temperatures the energy loss was propor-tional to about the fifth power of the temperature. This result could only be explained if hydrogen at high temperatures were dissociated into atoms. The diffusion of the hydrogen atoms from the filament, and their recombination at a distance from it, would cause an enormous increase in heat conduction. After publishing these preliminary results, he was naturally very interested in getting more information about the properties of these hydrogen atoms. A large number of experiments, extending over several years, were thus made in this study of atomic hydrogen. Nearly all of these experiments would have seemed quite useless, or even foolish, to a man who was making a direct and logical attack on the problem of improving tungsten lamps. When nitrogen at low pressure was introduced into a bulb containing a tungsten filament at extremely high temperatures, such as 2800oK., the nitrogen disappeared at a rate which was independent of its pressure. This suggested that the reaction velocity was limited by the rate at which the tungsten evaporated from the filament. To check this hypothesis the rate of loss of weight of filaments at various temperatures was measured in good vacuum. This rate varied with the temperature in accordance with known thermodynamic laws, and it was concluded that the loss of weight was really due to evaporation and not to chemical action of residual gases or to electric currents that passed from the filament to the surrounding space. A comparison of the rate of disappearance of nitrogen with the loss of weight in the filament showed that one molecule of nitrogen disappeared for every atom of tungsten that evaporated. A brown compound, WN2, was formed which deposited on the bulb and decomposed when water vapor was introduced, forming ammonia gas. From time to time the question kept arising-how good would a lamp be if it had a perfect vacuum? And now, from his studies, he began to have an answer. Hydrogen, oxygen, nitrogen, carbon monoxide, and in fact every gas that he introduced, with the exception of water vapor, did not produce blackening of the lamp bulb. The serious blackening that occurred with only small amounts of water vapor depended upon a cyclic reaction in which atomic hydrogen played an essential part. The water vapor of molecules in contact with the hot filament produced a volatile oxide of tungsten and the hydrogen was liberated in atomic form. The volatile oxide deposited on the bulb where it was reduced to the metallic state by the atomic hydrogen, while the water vapor produced returned to the filament and caused the action to be repeated indefinitely. Thus, a minute quantity of water vapor may cause a relatively enormous amount of tungsten to be carried to the bulb. The question then arose whether the traces of water vapor, which might still exist in a well-exhausted lamp, were responsible for the blackening which limited the life or the efficiency of many of these lamps. They made some tests in which well-made lamps were kept completely immersed in liquid air during their life, so that there could be no possibility of water vapor coming in contact with the filament. The rate of blackening, however, was exactly the same as if no liquid air had been used. Having thus proved that the blackening of a well-made lamp was due solely to evaporation, he could conclude with certainty that the life of the lamp would not be appreciably improved even if they could produce a perfect vacuum.
The heat of Hydrogen disassociation
Early in 1911 William Stanley, one of the pioneers in the electrical industry, felt that General Electric should do more fundamental work in connection with heating devices. Since Langmuir had become interested in the physics of heat losses from filaments in gases, he was glad to work along these lines and therefore undertook to direct a small laboratory at Pittsfield, Mass. at which he spent about two days a week. Besides studying the heat losses from plane surfaces at various temperatures he measured the heat losses from wires of various sizes in air at different temperatures, working at first with platinum wires, and was able to develop a theory of the heat losses which enabled him to calculate the loss from a wire of any size at any temperature in any gas, provided of course, that the gas did not dissociate at high temperatures. Having now a definite theoretical basis on which to calculate the normal heat loss by convection, he was able to prove that the abnormal rate of heat loss previously observed with tungsten filaments at high temperatures in hydrogen was due to actual dissociation; in fact he was able to calculate the heat of dissociation and the degree of dissociation at different temperatures. In order to confirm these conclusions, he undertook experiments with heated tungsten wires in mercury vapor at atmospheric pressure to measure heat losses under such conditions. A little later he experimented with nitrogen and found that nitrogen did not dissociate either. In both of these gases the filaments could be maintained at temperatures close to the melting point for a far longer time than if heated in vacuum at the same temperature. Thus the rate of evaporation was greatly decreased by the gas, many of the evaporating tungsten atoms being brought back to the filament after striking the gas molecules. By this time he was familiar with all the harmful effects which gas can produce in contact with filaments and knew under what conditions these bad effects could be avoided. In particular, he realized the importance of avoiding even almost infinitesimal traces of water vapor. Thus, when he found a marked effect of mercury vapor and nitrogen in reducing the rate of evaporation, it occurred to him that it might be possible to operate a tungsten filament in gas at atmospheric pressure and obtain a long useful life. Of course, it would be necessary to raise the temperature far above that at which the filament could be operated in vacuum in order to compensate for the serious loss in efficiency due to convection. Whether or not the increased rate of evaporation, due to this increase in temperature, would be more important than the decrease in the rate due to the gas was a matter that could only be tested by experiment. After a series of detailed experiments, they were soon able to make lamps having a life of over 1000 hours with an efficiency about of 80 to 40 per cent better than could have been obtained with filaments in vacuum. A result out of which, General Electric made astronomic fortunes.
Things learnt in pursuit of studying atomic hydrogen
The invention of the gas-filled lamp is thus nearly a direct result of experiments made for the purpose of studying atomic hydrogen. Langmuir had no other objective in view when he first heated tungsten filaments in gases at atmospheric pressure. Even at the time that he made these experiments at higher pressures, they would have seemed to him useless if his prime objective had been to improve the tungsten lamp. However, as it turned out, this preliminary work, not only produced a superior lamp but also resulted in the following important conclusions:
- The energy loss from heated wires in various gases can be readily calculated by simple equations. For nitrogen and mercury vapor the results calculated in this way agree well with experimental results up to temperatures as high as 3500oK.
- With air and carbon dioxide similar agreement was obtained up to the melting point of platinum.
- In the case of hydrogen, however, there was agreement only up to about 2100oK. Above that the energy loss increased extremely rapidly such that at 3300oK it was four or five times the calculated value.
- This was explained by assuming that hydrogen at very high temperatures is dissociated into atoms.
- A theory of the heat conductivity of a dissociating gas was developed.
- The heat of reaction and the degree of dissociation at various temperatures was thus calculated. Experiments at low pressures proved that the phenomenon was a true dissociation, and that the volume of the dissociation products was approximately twice the volume of the original hydrogen.
That is, the dissociation took place according to the equation  .
.
- There was much evidence that the dissociation was not electrolytic.
- Nitrogen, even at 3500oK, was not dissociated at atmospheric pressure. That is, its dissociation did not exceed 5%, at 3500oK.
- The following quantitative results were obtained. The heat of reaction at constant volume for 2H H2 was 550,000 joules, or 130,000 calories. At constant pressure it was approximately 575,000 joules, or 136,000 calories at 3000oK.
- The degree of dissociation at any temperature, T, was given by the equation
- Here p1 is the partial pressure of the hydrogen atoms (in atm), and P is the total pressures (in atm). From this equation the free energy of formation of hydrogen molecules from atoms can be calculated.
Atomic hydrogen flames
Armed with fresh knowledge, Langmuir continued his work exploring the new phenomenon of Atomic Hydrogen and found that the heat carried away from an incandescent wire by a surrounding inert gas at ordinary temperatures increases roughly in proportion to the 1.9th power of the absolute temperature, T of the filament. This relation holds, for example, for such gases as nitrogen, argon, and mercury vapor up to the temperature of melting tungsten, 3660oK.
In the case of hydrogen, however, abnormal results were obtained in experiments made at high temperatures. Up to about 1700oK the normal exponent of 1.9 was observed, but at higher temperatures the exponent increased until at 2600oK and above it was about 5.0. At 3400oK the heat conducted by hydrogen was twenty-three times as high as that carried by nitrogen under similar conditions.
Nernst in 1904 had developed the theory of heat conduction in a dissociating gas and had shown that dissociation results in a great increase in the heat conductivity. The dissociation products diffuse from the hot portions of the gas into the cold portions and there, by recombining, give up the large energy of the reaction. This suggested, as mentioned earlier that the abnormal heat conductivity of hydrogen at high temperatures was due to dissociation of the hydrogen into atoms according to the reaction. By means of theoretical considerations it was possible to determine the degree of dissociation and the heat of the reaction by which atoms combine to form molecules. The results that were published in 1915 gave 90,000 calories as the heat of combination of 2 grams of hydrogen atoms at constant pressure and at 3000oK. The degree of dissociation, x, expressed as the fraction of the molecules which have been dissociated, was found to be, at atmospheric pressure, 0.00165 at 2000oK, 0.0109 at 2400oK, and 0.0421 at 2800oK. Niels Bohr, one of the most prominent theoretical physicist of the time and a close friend of Langmuir, calculated in a valuable and wonderfully suggestive paper on the Constitution of Atoms and Molecules (Phil. Mag. xxvi, p.863, 1913), the heat of formation of hydrogen molecules from the atoms to be 60,000 calories per gram-molecule. He pointed out that this value was considerably less than the value of 130,000 found by Langmuir (J. Amer. Chem. Soc. xxxiv, p. 860, 1912) by measuring the heat-conduction through the gas from an incandescent wire in hydrogen. In order that the results obtained by Langmuir should not bear false evidence against the theory derived by Dr. Bohr, he gave a preliminary account of some more recent measurements carried out by Mr. G.M.J. Mackay and himself, on the dissociation of hydrogen. The method adopted was similar to that previously used, namely, to determine the heat-loss from electrically heated tungsten wires in hydrogen. In these experiments, however, very much greater care was used in determining the temperatures of the wires and in obtaining extremely pure hydrogen. Pressures of hydrogen ranging from 1 mm up to 760 mm of mercury were used. Still further experiments were made at pressures from 0.01 mm up to 20 mm pressure. The measurements at low pressures gave very interesting results: At about one-tenth of an atmospheric pressure in hydrogen, the total heat-loss from filaments heated to very high temperatures (3000oK) was several times as great as in hydrogen at atmospheric pressure. By subtracting the heat-loss due to normal heat-conduction from the total observed heat-loss, the heat carried by diffusion of hydrogen atoms was obtained. By plotting the logarithm of this quantity against the reciprocal of the absolute temperature, straight lines were obtained in each experiment. The slopes of all these lines were practically equal, no matter what pressure of the hydrogen was used (above 10 mmHg). On the assumption that the diffusion coefficient varies with the 3/2 power of the temperature, this lead to the result that the heat of formation of hydrogen is about 76,000 calories per gram-molecule, as against 130,000 previously found. The reason for the high value of the latter figure is that it was based on a calculation of the actual value of the diffusion coefficient of hydrogen atoms through ordinary hydrogen. These results showed that the actual degree of dissociation was much smaller than that previously found, and that even at 3500oK hydrogen was probably not dissociated to a very great extent. Further measurements finally suggested the heat of recombination to be 90.000 calories per gram/molecule. Still a value of extraordinary proportions.
Atomic hydrogen flames
Chemical Properties of Atomic Hydrogen
While the measurements of the heat losses from filaments in hydrogen were being made, other experiments showed that hydrogen which had been in contact with heated filaments acquired entirely new chemical properties, and they were quite in accord with those to be expected of an atomic form of the element. A very remarkable phenomenon occurred when a mixture of oxygen and hydrogen at low pressure was admitted to a bulb containing a filament at 1500oK. The oxygen reacted with the filament rapidly to form W03, which evaporated at this temperature as fast as formed. The oxygen thus cleaned up at a rate proportional to its own pressure and the pressure of oxygen thus fell to half value about every 2 minutes in a bulb of ordinary size. All this occurred exactly as if no hydrogen were present. During this time there was no measurable disappearance of hydrogen. After 10 or 15 minutes the oxygen was nearly all gone and then for 5 or 10 minutes more the gas pressure remained apparently constant and corresponded exactly to that of the hydrogen which was admitted. Then suddenly, when the pressure of oxygen was low enough (10-9 mm), the hydrogen pressure began to drop by dissociation and in a few minutes the pressure fell practically to zero. Water vapor has an effect similar to oxygen in preventing the dissociation of hydrogen. At filament temperatures of 1750oK some of the hydrogen disappeared while the oxygen was cleaning up, but the kink in the curve still occurred when the oxygen was gone. Water vapor and oxygen are thus powerful catalytic poisons for the reaction of the hydrogen dissociation.
Arcs in Hydrogen at Atmospheric Pressures
Several years earlier in the General Electric laboratory studies were made of arcs between tungsten electrodes in various gases. Arcs in hydrogen were remarkable because of the high voltage drop and small cross section. A 10-ampere, direct-current arc between heavy tungsten electrodes about 7 mm apart in a bulb containing pure hydrogen at atmospheric pressure appeared as a sharply defined, brilliant red line about 0.5 mm in diameter along which the potential gradient was 150 volts per centimeter, making a power dissipation of 1500 watts per centimeter of length, this being about fifteen times as great as in nitrogen or argon. This abnormal behavior of hydrogen was then attributed to the dissociation which apparently carried energy so rapidly out of the arc.
Arcs in Hydrogen at Low Pressures
In attempting to obtain the Balmer spectrum of hydrogen without contamination by the secondary spectrum, they built very long vacuum tubes of moderate bore, in which they passed currents as large as 20 amperes through moist hydrogen at about 0.5 mmHg pressure. They observed many remarkable phenomena. Short pieces of tungsten wire projecting into the discharge were heated to incandescence, although a fine thread of glass or a platinum wire in a similar position was apparently not heated by the discharge. On drying the hydrogen with phosphorus pentoxide the secondary spectrum (due to molecular hydrogen) appeared strongly and the Balmer spectrum (due to atomic hydrogen) nearly disappeared. The heating of the tungsten wire was also prevented by drying the hydrogen. In correspondence with Professor Wood, Langmuir pointed out that oxygen and water vapor decrease the rate of dissociation of hydrogen in contact with tungsten and must thus also tend to prevent the recombination of hydrogen atoms an a tungsten surface. He also suggested that moisture poisons the catalytic activity of the dry glass surfaces that otherwise converts atomic into molecular hydrogen. Thus with moist hydrogen the tube becomes filled with nearly pure atomic hydrogen and the diffusion of this to the catalytically active tungsten wire causes the heating of the latter. Calculations based on the measured heat of dissociation proved that a pressure of only 0.16 mmHg of atomic hydrogen at 5000C would suffice to maintain a tungsten filament at 2400oK. These conclusions were confirmed by Wood's observations that the walls of the tube became only slightly heated if the hydrogen was moist, whereas they were strongly heated with dry hydrogen. A tungsten wire was heated red hot even when mounted in a side tube (of 5 mm diameter) at a distance of 4 cm from the discharge tube, showing that the hydrogen atoms could diffuse in relatively large quantities out of the discharge. It occurred to Langmuir that it should be possible to obtain even higher concentrations of atomic hydrogen by passing powerful electric arcs between tungsten electrodes in hydrogen at atmospheric pressure and this atomic hydrogen could be blown out of the arc by a jet of molecular hydrogen directed across the arc.
Preliminary Experiments with Flames of Atomic Hydrogen
To try out the possibility of blowing atomic hydrogen out of an arc, 20-ampere arcs from a constant-current transformer were passed between two tungsten rods 6 mm in diameter mounted transversely in a horizontal alundum tube (10 cm diameter) through which a stream of hydrogen flowed. With voltages from 300 to 800V, arcs could be maintained with electrode separations up to 2 cm. The magnetic field of the arc caused the hydrogen to move transversely so that it became fan-shaped. Iron rods 2 or 3 mm in diameter melted within 1 or 2 seconds when they were held 3 to 5 cm above the arc. By directing a jet of hydrogen from a small tube into the arc, the atomic hydrogen could be blown out of the arc and formed an intensely hot flame. To maintain the arc in a stable condition the electrodes were brought close together (1 to 3 mm), but the arc did not remain entirely between the electrodes, but extended as a fan to a distance of 5 to 8 mm. The flame of atomic hydrogen, however, extended far beyond the arc. At distances of 1 or 2 cm from the arc molybdenum (m.p. 2900oK) melted with ease. Near the end of the arc tungsten rods and even sheet tungsten (m.p. 3660oK) could be melted. The use of hydrogen under these conditions for melting and welding metals proved to have many advantages. Iron can be melted without contamination by carbon, oxygen, or nitrogen. Because of the powerful reducing action of the atomic hydrogen, alloys containing chromium, aluminum, silicon, or manganese can be melted without fluxes and without surface oxidation.
Temperature of Atomic Hydrogen Flame
Compared with Other Flames Let us suppose we could obtain atomic hydrogen in bulk at atmospheric pressure and room temperature and that we could then let this burn to the molecular form in a flame. What would be the temperature of this flame and how would it compare with that of other flames? Taking the heat of reaction (for 2 grams) to be 98,000 calories and taking the specific heat of molecular hydrogen (for 2 grams) to be 6.5 + 0.0009 T, we find that the heat of the reaction would be sufficient to heat the hydrogen to 9200oK. The dissociation of the hydrogen, however, would prevent the temperature from rising to any such high value. If x is the degree of dissociation at the maximum temperature reached, the available heat of recombination is only (1-x) 98,000. Langmuir plotted two curves based on his accumulated data . These two curves intersected at T = 4030oK and x = 0.642. Thus atomic hydrogen at room temperature and atmospheric pressure would heat itself to 40300K and the degree of dissociation would then be 0.642. There is another factor which tends greatly to increase the temperature of the atomic hydrogen flame even above the calculated value of 4030oK. The atomic hydrogen, instead of being originally at room temperature, is already at a high temperature at the moment of its escape from the arc. The conditions are analogous to those in an oxyhydrogen flame in which both gases are preheated. Thus the upper limit of temperature is fixed only by the degree of dissociation of the hydrogen and the rate at which heat is lost by radiation or contact with bodies of lower temperature.
Rate of Surface Heating by Flames
Although the high temperature of the atomic hydrogen flame is of great importance when it is desired to melt substances of very high melting point such as tungsten, a factor of even greater importance in for example ordinary welding operations is the speed with which heat can be delivered to a surface per unit area. If a Bunsen burner flame delivers 51 watts per sq. cm to the whole surface of a black body, it would thus heat it to a maximum temperature of 17300K. If heat is applied by the flame to one side of a plate-shaped body and the heat is radiated from both sides, the maximum temperature reached would be 1450oK. The fact that the Bunsen flame does not heat bodies so hot as this indicates that the rate of surface heating decreases as the temperature of the body increases. The heat reaches the surface from such a flame by conduction through a relatively stationary film of gas. The decrease in the temperature gradient when the body becomes hot would explain the lower rate of surface heating. With 1330 watts per sq. cm delivered by the atomic hydrogen flame, the temperature of a black body would rise to 3900oK. The power radiated from tungsten at its melting point is 395 watts per sq. cm, and 1330 watts per sq. cm should heat tungsten to about 5300oK. At such high temperatures, however, the rate of surface heating by an atomic hydrogen flame must decrease because of the fact that the hydrogen remains partly dissociated so that the recombination is not complete. With surface temperatures below 2000oK, however, this factor would be negligible. It is probable that the rate of surface heating would be dependent not so much on the temperature gradient in the surface film of gas as on the rate of diffusion of atomic hydrogen through this film. Thus we may expect the rate of delivery of energy to a metal surface to remain nearly constant until the surface reaches a temperature of at least 2000oK.
Total Heat Delivered to Surfaces
It now became of interest to determine what fraction of the total energy in an arc or a flame could be delivered to a large flat surface against which the flame was directed. For this purpose a cylinder of copper 10.5 cm in diameter and 9.8 cm long was used, which weighed 7950 grams. The flame was directed against one of the flat polished ends, and the rate of temperature rise was measured. An atomic hydrogen flame was produced by a 60-ampere a.c. arc using a torch like that shown here:
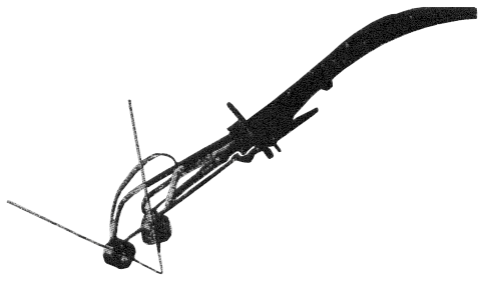
The voltage across the electrodes was 70 volts. A wattmeter showed that the power consumption in the arc was 3510 watts, which gives a power factor of 0.84. The electrodes were tungsten rods 3.2 mm in diameter which made an angle of 55 degrees with one another. The rate of flow of hydrogen which bathed the electrodes was 14.2 liters per minute (30 cubic feet per hour). From the temperature rise of the copper block the heat delivered to the surface was found to correspond to 3100 watts when the electrode tips were 3 to 5 mm from the copper surface. This decreased to 2800 watts at 13 mm, 2500W at 25 mm, and 2200W at 35 mm. With the arc turned off but the molecular hydrogen burning in the air, the rate of heating corresponded to 250 watts with the electrodes 6 mm from the surface. Subtracting this energy delivered by the combustion of the hydrogen in the air, we find that the energy carried to the metal by the atomic hydrogen ranged from 2850 to 1950 watts. Thus with the electrodes 3 mm from the metal 82 per cent of the power input into the arc was delivered to the copper surface. This efficiency became 78 per cent at 6 mm, 71 at 13 mm, 65 at 25 mm, and 55 per cent at 35 mm. The power corresponding to the complete combustion of 14.2 liters of hydrogen per minute is 2360 watts. Actually, only 250 watts or 11 per cent of this reaches the copper. The total energy of the arc and the flame of molecular hydrogen is 5870 watts, of which 3100 watts or 53 per cent is delivered to the copper. An oxy-acetylene flame from a standard welding torch consuming 30.6 liters of oxygen per minute (64.8 cubic feet per hour) and 28.6 liters of acetylene per minute (60.6 cubic feet per hour) delivered energy at the rate of 4400 watts to the copper surface. A smaller torch consuming 13.7 liters of oxygen per minute and 13.0 of acetylene (29.0 and 27.5 cubic feet per hour, respectively) gave energy to the copper at the rate of 3900 watts.
Application of Atomic Hydrogen Flames to Welding of Metals
The high temperature of The Atomic Hydrogen flame, together with its powerful chemical reducing action and the avoidance of gases containing oxygen and nitrogen, render it particularly useful for welding, not only for iron and its alloys, but for such metals and alloys as contain aluminum, magnesium, chromium, manganese, etc. The previous figure illustrates one of the later forms of torch used for welding. Two tungsten rods, as electrodes, are held at a definite angle to one another by easily adjustable clamps, and a jet of hydrogen is directed from a small nozzle along each of these rods near its end. The hydrogen thus bathes the heated parts of the electrodes and forms a gentle blast of gas which passes through the arc between the electrode tips, and blows the atomic hydrogen away from the electrodes so that these are not unduly heated. Other torches were also built suitable for automatic welding using machine feed. The electrodes were ordinarily separated 3 or 4 mm and the arc assumed a fan shape extending 6 to 10 mm from the electrodes. Alternating current was generally used. To utilize the atomic hydrogen flame for the welding of metals it became important to have easy and complete control of the flame. Many different forms of welding torches were constructed and tested. The electrodes between which the arc passed were mounted at a convenient angle to one another and were adjustable so that they could be brought into contact at a point which was exposed to a blast of hydrogen from one or more orifices. Thus the atomic hydrogen was blown out of the arc in a definite direction and formed a flame which could be brought into contact with the metal to be welded. The jet of hydrogen also served to bathe all the heated parts of the electrodes and the work, thus preventing oxidation and the introduction of impurities such as nitrogen into the weld. The hydrogen was supplied by a tube which passed through the handle and then by flexible tubes was delivered to each of the electrode holders and escaped through the annular spaces between the electrodes and the lava insulators. Sufficient hydrogen was used not only to surround each of the electrodes to their tips but to form a blast which blew the atomic hydrogen against the work and bathed it in hydrogen.
Electrical Apparatus
Both the striking voltage and the arc voltage were higher for an arc in hydrogen than for the ordinary welding arc since there was no appreciable amount of metallic vapor generated in the arc. The standard arc welding equipment of those days was therefore not suitable as a power source for operating the atomic hydrogen torch. If direct current was used the arc could be stabilized by a series resistance or a specially designed generator of the constant-current type could also be used. With series resistance a line voltage of 250 was found to give good results. Alternating current was more convenient and, since the arc could then be stabilized by reactance instead of resistance, greater efficiency was usually obtained. A line voltage of 350 to 400 gave satisfactory operation. Voltages as high as this were needed solely to give stability and to enable the arc to be started at any time by separating the electrodes even when these were cold. A number of tests were made to determine the voltages required to strike an arc by means of the lever mechanism of the torch. With cold electrodes an open circuit ac voltage of 320 was needed for striking the arc. After the arc had been started and the electrode tips had reached the operating temperature a line voltage of only 150 was sufficient to restart the arc as long as the electrodes remained nearly at the operating temperature. These lower voltages could also be employed to start and maintain the arc if the electrodes were first raised to high temperature by a high current while separating them very slightly so as to have a high contact resistance or by bringing a thin tungsten rod between the separated tips of the electrodes. It was more convenient, however, to use open circuit voltages of approximately 400V rather than to employ these special means of starting the arcs. In normal operation the drop across the arc was in the neighborhood of 80 volts. To avoid danger to the operator the entire arc circuit was preferably insulated from ground. A motor-generator was used to give either direct or alternating current for the arc, but it was usually more satisfactory to use a specially designed transformer. The connections that were used in most of the work to be described are shown in Fig. 3. When the arc was not operating the electrodes were in contact by the action of the spring attached to the control lever, so that there were no voltages on the electrodes and the torch could be laid down on any material without danger of flashing the operator's eyes. To strike the arc the electrodes were merely separated by pressing the lever. Should the open-circuit voltage at any time be impressed across the electrodes when separated, or the operator break the arc by spreading the electrodes too far apart, a relay in the arc circuit (contactor B as shown in Fig. 3) would trip the feeder circuit, in which case it was necessary for the electrodes to be brought in contact again before the main feeder circuit could be restored.

The voltage drop across the arc while in operation varied from 60 to 100 volts, depending on the amount of opening between the electrode tips. This voltage was nearly independent of the current, between the limits of 20 and 70 amp, although a slight decrease in voltage was usually noted when the current was increased. Repeated experiments showed that the lower voltage arc (60 to 80 volts) obtained by separating the electrodes only 1/16 or 1/8 of an inch had a more concentrated working zone and was the most efficient arc for most kinds of welding. By bringing the arc closer to the surface of a larger mass of metal it was found that the metal melted very rapidly. For welding, the maximum rate of heating was desired and this was obtained by bringing the torch so close to the metal that the lower portion of the fan shaped arc was just about in contact with the metal and this caused the arc to change its shape somewhat. The tips of the electrodes were then usually about 3/8 to 1/2 in. from the metal. Portions of the arc could at times become short-circuited by the metal so that the tracks of cathode spots on the metal could be seen,but this seemed to play no important part in the welding process.
Conclusions
The above is a general account of the course of events that led to the development of the atomic hydrogen arc welding technique. Although Langmuir himself had many ideas for other applications of atomic hydrogen, such as using it in melting furnaces, and although General Electric themselves were aware of the vast potential of atomic hydrogen (as expressed by the editor of the General Electric Research Laboratory Publication). The point of inception of many important practical processes can be found in researches in pure science. Following Dr. Langmuir's discovery of atomic hydrogen, conclusion was reached that flames of this gas make possible new applications of far-reaching importance), no subsequent effort was made to develop neither the welding method nor any other application using atomic hydrogen. In spite of the obvious value of the process, industry's excuse for laying the process aside was that it had been replaced by better processes such as Heliarc, TIG, and MIG welding, though plasma arc welding is rarely mentioned which has also almost disappeared from the market. Since plasma arc welding is merely an extension of the atomic hydrogen process, the reasons are undoubtedly the same. As a welding process, atomic hydrogen arc welding was obsolesced by MIG and TIG neither of which compare to its welding efficiency and uses. Considering that atomic hydrogen arc welding hardly got of the ground before it was replaced, it is not a far fetched thought to assume that the interests of welding suppliers and electric power companies were being nursed so that more archaic tanks, transformers, gauges, torches, electrodes, gases, fluxes, power etc. could continue to be sold at profit. The reader is reminded of the fact that Langmuir's experiments and findings were taking place almost 100 years ago. Since then, technology has progressed dramatically in many fields. If Langmuir had at his disposal the know-how and technology of today, our world may have looked different. However, we are now in the fortunate situation that we do possess the data of Langmuir's findings, which can be combined with today's technological know-how. The obvious direction in which to look is the same direction that seems to have been the fundamental reason for Langmuirs discoveries having been swept under the carpet: CLEAN AND ABUNDANT ENERGY. If anyone looks up to find any information on atomic hydrogen arc welding, only a few lines will appear, simply informing that the arc is maintained between two metal electrodes in an atmosphere of hydrogen. Shielding is obtained from the hydrogen. Pressure and/or filler metal may or may not be used. Although the process has limited industrial use today, atomic hydrogen welding is used to weld hard-to-weld metals, such as chrome, nickel, molybdenum steels, Inconel, Monel and stainless steel. Its main application is tool and die repair welding and for the manufacture of steel alloy chains. Also used in special military welding requirements. Nothing is mentioned of the extraordinary properties of atomic hydrogen, nor of its potential for the use as energy to drive the wheels of mankind. However, and quite surprisingly, in one edition of Van Nostrand's Encyclopedia of Science it was stated Hydrogen molecules dissociate to atoms endothermically at high temperatures (heat of dissociation about 103 cal/gram mole) in an electric arc, or by irradiation. The hydrogen atoms recombine at the metal surface to provide heat required for welding. What is surprising here is that the actual energy value needed for the dissociation of the hydrogen molecule is given, but the calorific value for the recombination of the atoms into molecules is strangely omitted. From Langmuir's experiments and findings we know that the minimum calorific value for the recombination of atoms was agreed to be in the region 90.000 cal/gram molecule. In other words we have an input energy of 103 cal/gram molecule and an output energy of 90.000 cal/gram molecule. In conventional science this seems to be violating the law of conservation of energy. Langmuir explained this (however, not very convincingly) by the heat being carried forward from the arc to the metal surface. One area which certainly deserves the attention of modern science, is the replication of Langmuirs experiments using high-tech measurement equipment.
Heat, ZPE & Cold Fusion
During the 1920s there was a general scientific consensus in the community of theoretical physics, that the space between the nucleus of the atom and its orbiting particles, was empty space, vacuum. It was not before the late 1950s that the existence of Zero Point Energy was discovered by the Dutch physicist M. J. Sparnaay. He continued the experiments carried out by Hendrick B. G. Casimir in 1948 which showed the existence of a force between two uncharged plates that arose from electromagnetic energy surrounding the plates in a vacuum. Mr. Sparnaay discovered that the forces acting on the plates arose from not only thermal energy (heat) but also from another type of radiation now known as classical Zero Point Energy. Mr. Sparnaay determined that not only did the zero point electromagnetic energy exist in a vacuum but also that it persisted even at a temperature of absolute zero. The term Zero Point Energy (ZPE) has been based on the concept that even if matter were cooled down to absolute zero (minus 273oC), in terms of its temperature, the energy field still remains. Because it exists in a vacuum, ZPE is homogeneous (uniform) and isotropic (identical in all directions) as well as ubiquitous (exists everywhere). In addition, the intensity of the energy at any frequency is proportional to the cube of that frequency. Consequently, the intensity of the energy field increases without limit as the frequency increases resulting in an infinite energy density for the radiation spectrum. With the introduction of the ZPE into the classical electron theory, a vacuum at a temperature of absolute zero is no longer considered empty of all electromagnetic fields. Instead, the vacuum is now considered as filled with randomly fluctuating fields having the ZPE spectrum. The special characteristics of ZPE are, as mentioned above, that it has a virtually infinite energy density and that it is ubiquitous (even present in outer space), which makes it very desirable as an energy source. However, because high energy densities exist at very high frequencies, and because conventional methods are only able to convert or extract energy efficiently at lower frequencies, effectively tapping this energy source has been unavailable using conventional techniques.Until now. Had Langmuir been familiar with ZPE, he would most certainly have reached other conclusions in terms of explaining the extraordinary energy properties of atomic hydrogen. As it was proven by Langmuir, the volume of the hydrogen when dissociated into atoms increases to the double of the volume of its molecular state. Upon recombination, heat energy is released to the tune of 90.000 cal/gram molecule. When incorporating ZPE in the explanation of the hydrogen process, it could be argued that the hydrogen is not really a fuel but rather a medium, gateway or a super-conductor of ZPE from the vacuum of space, converting ZPE radiation and ultra-high frequency electrical energy into infrared (heat) radiation. On recombination into molecules the ZPE is squeezed out, releasing the absorbed energy. Actually since heat is infra-red spectrum radiation, the process can be conceived as a means of converting ZPE from an ultra-penetrating cold spectrum radiation, to a mass-reactive infra-red heat spectrum radiation, and that is the proximate source of so called FREE ENERGY, in the form of exothermic heat radiation. ZPE can be analogized to a concentrate of sunshine, except it penetrates all matter all the time and is not affected by day or night, so it can be converted into usable energy at all times with the appropriate technology, such as the atomic hydrogen process. The apparent source of the anomalous exothermic heat produced in Cold Fusion is also based on atomic hydrogen. It is important to note that the hydrogen process does not involve a consumption of the hydrogen as it is not combusted in the process. It is merely dissociated and recombined and can therefore be recycled over and over again without consuming more hydrogen than the quantity used to start with.

 .
.
 The voltage drop across the arc while in operation varied from 60 to 100 volts, depending on the amount of opening between the electrode tips. This voltage was nearly independent of the current, between the limits of 20 and 70 amp, although a slight decrease in voltage was usually noted when the current was increased. Repeated experiments showed that the lower voltage arc (60 to 80 volts) obtained by separating the electrodes only 1/16 or 1/8 of an inch had a more concentrated working zone and was the most efficient arc for most kinds of welding. By bringing the arc closer to the surface of a larger mass of metal it was found that the metal melted very rapidly. For welding, the maximum rate of heating was desired and this was obtained by bringing the torch so close to the metal that the lower portion of the fan shaped arc was just about in contact with the metal and this caused the arc to change its shape somewhat. The tips of the electrodes were then usually about 3/8 to 1/2 in. from the metal. Portions of the arc could at times become short-circuited by the metal so that the tracks of cathode spots on the metal could be seen,but this seemed to play no important part in the welding process.
The voltage drop across the arc while in operation varied from 60 to 100 volts, depending on the amount of opening between the electrode tips. This voltage was nearly independent of the current, between the limits of 20 and 70 amp, although a slight decrease in voltage was usually noted when the current was increased. Repeated experiments showed that the lower voltage arc (60 to 80 volts) obtained by separating the electrodes only 1/16 or 1/8 of an inch had a more concentrated working zone and was the most efficient arc for most kinds of welding. By bringing the arc closer to the surface of a larger mass of metal it was found that the metal melted very rapidly. For welding, the maximum rate of heating was desired and this was obtained by bringing the torch so close to the metal that the lower portion of the fan shaped arc was just about in contact with the metal and this caused the arc to change its shape somewhat. The tips of the electrodes were then usually about 3/8 to 1/2 in. from the metal. Portions of the arc could at times become short-circuited by the metal so that the tracks of cathode spots on the metal could be seen,but this seemed to play no important part in the welding process.
